Although plastics (polymers) have found widespread applications, their low thermal conductivity (i.e. being poor heat conductors) has hindered their use in fields such as microelectronics, LED light and battery encapsulations, automobiles and heat exchangers where they would have proven to be highly beneficial owing to their light weight, corrosion resistance and ease of processing.
Low thermal conductivity of polymers arises from their molecular structure composed of long spaghetti-like chains, composed of connected molecules (called monomers), inter-twined with and coiled onto one another resulting in entanglements, defects and voids. Although these chains have strong covalent bonds along their backbone, the interactive force between chains is the very weak van der Waals (VDW) force. Overall, this molecular make-up of polymers results in dissipation of the heat carrying vibrations (called phonons) in the material resulting in very low thermal conductivity. This is similar to the difficulty of forming waves or vibrations on a loosely hung string as compared to a taut string.
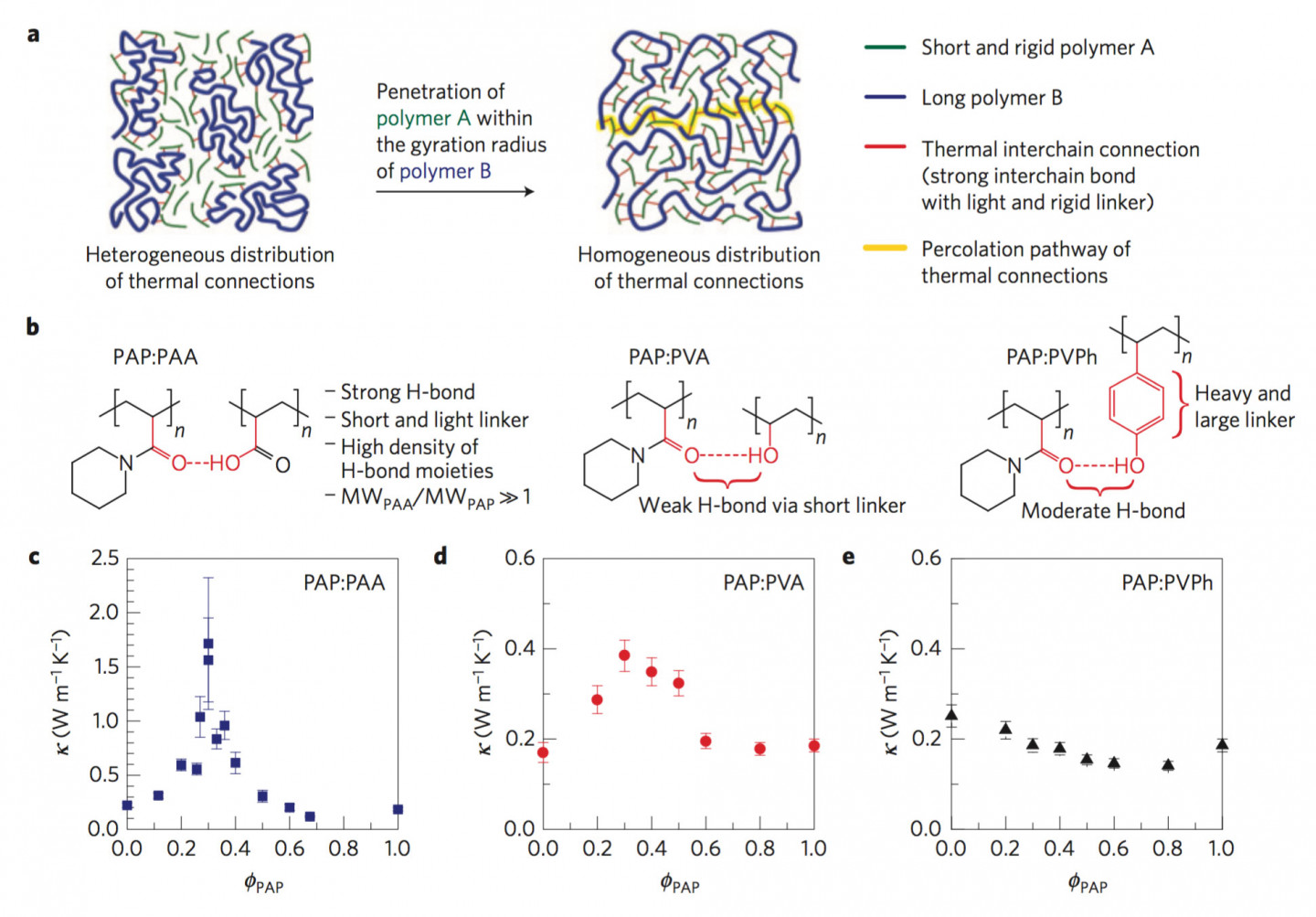
To overcome this thermally inefficient molecular structure of plastics, a team at the University of Michogan envisioned replacing the weak VDW interactions between polymer chains with hydrogen bonds which are 10-100 folds stronger. Moreover, such inter-chain connections should, firstly, be made via short linker units to reduce the probability of phonon dissipation and, secondly, be present in high enough concentration to provide a continuous path to a propagating phonon (Fig. 1).
The three design parameters to enhance thermal transport were met by designing a material system consisting of two different polymers: one, a short chain stiff polymer (Poly(N-acryloyl piperidine), PAP) interpenetrating into the second long chain flexible polymer (Polyacrylic acid, PAA) matrix and holding it in a more extended conformation through strong hydrogen bonds. The polymers were mixed in certain ratios of monomers of PAP and PAA in an organic solvent and thin polymer blend films were made by spin-casting.
The highest thermal conductivity () measured was 1.72Wm-1K-1 for the monomer molar ratio of 30% PAP which is one order of magnitude higher than that of individual polymers: PAP ( = 0.19Wm-1K-1) and PAA ( = 0.22Wm-1K-1). Furthermore, we characterized and confirmed that the films with the highest had a very high concentration of H-bonds resulting in mixing of two polymers at the molecular level and, therefore, in extended chain configuration (like a taut string). This work was the first to show molecular engineering of polymers to enhance their heat transport properties.
For more information, please visit http://ns.umich.edu/new/multimedia/slideshows/22539-heat-conducting-plastic-developed-at-u-michigan